Episodic memory, pattern separation, and the hippocampus
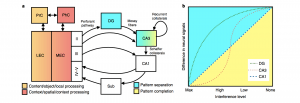
A critical component of episodic memory is the ability to overcome interference from overlapping or similar experiences such that they can be stored independently. Pattern separation, or the process of disambiguating similar events into distinct representations, is one computation the hippocampus is able to perform to serve this function of episodic memory. Our recent review on hippocampal pattern separation (Leal & Yassa, Nature Neuro, 2018) discusses advances in the field and suggests guidelines for future research using pattern separation as a construct that can aid understanding of both memory processes and brain disease.
Factors that modulate episodic memory
Emotional modulation of episodic memory
A major focus of the lab is how the emotional modulation of memory serves to enhance or impair memory for important experiences. Typically, episodic memory is thought of in terms of three main components: what, where, and when. However, one of the most important components of memory that often gets overlooked is why we remember. The significance of an experience is what creates a lasting memory. Understanding the dynamics of how significant memories are stored is crucial to uncovering how our memory system works, how this system may be altered in states of cognitive impairment, and how we might be able to target specific aspects of memory processing to either enhance or impair memory for certain experiences.
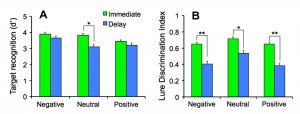
Cross-species evidence suggests that emotional memories are preserved (McGaugh, Annu Rev Neurosci., 2004), however, not all aspects of an emotional experience are better remembered. We developed an emotional mnemonic discrimination task that tests how emotion modulates memory using a pattern separation framework. We found a gist versus detail trade-off, where emotional details were forgotten while emotional gist was preserved (Leal et al., NLM, 2014). This task is sensitive to subtle behavioral alterations and can be used to examine the neural signals associated with accurate discrimination using high-resolution fMRI, which is necessary to understanding the neurobiological mechanisms underlying the emotional modulation of memory.
Memorability
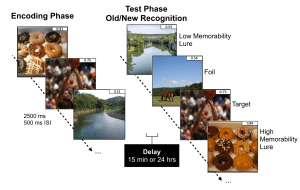
The intrinsic ability of certain elements to be consistently better remembered or forgotten by most people is known as memorability. Through the development of memorability-based mnemonic discrimination tasks that tax hippocampal pattern separation, we can analyze how memorability impacts episodic memory performance and how it interacts with other elements that impact why we remember and forget, such as emotional valence of experiences and lure image similarity.
Music’s impact on memory

Music plays a unique role in memory processing. We can recall music and memories associated with music well into old age, even in those with dementia. Music has the capability of inducing emotional arousal, which may provide a powerful approach toward the modulation of memory. Furthermore, examining other factors related to music such as perceived familiarity, pleasantness, and expertise may also play a role in music’s unique relationship with memory.
High-resolution structural and functional MRI
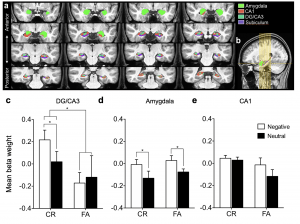
One goal of of the laboratory is to translate animal models of episodic memory, chronic stress, and age-related memory impairment to humans using high-resolution structural and functional MRI. Using mnemonic discrimination paradigms, we can investigate specific changes in hippocampal and amygdala dynamics using high-resolution fMRI in a comparative framework. We have found that the dentate gyrus (DG)/CA3 subfield of the hippocampus is sensitive to discriminating highly similar negative information over neutral information, while the amygdala is modulated by emotion regardless of the accuracy of discrimination (Leal et al., Hippocampus, 2014). We have also recently parsed the subregions of the amygdala to strengthen our hypothesis testing of animal models suggesting the basolateral amygdala is implicated in the modulation of memory (Leal et al., Hippocampus, 2017; Leal et al., Neurobiology of Aging, 2017).
We can now functionally image the whole brain at high resolution (1.5mm isotropic), which allows highly detailed hypothesis testing of MTL-cortical interactions during memory consolidation. These neural measures may be useful biomarkers of disease states, giving us a better understanding of targeted treatment options.
Task development
Development of tasks sensitive to MTL function has become an important direction for understanding disease states (e.g. A4 study, Sperling et al., Sci Transl Med, 2014). We have recently developed a novel mnemonic discrimination task that utilizes dynamic video stimuli of everyday experiences rather than static objects, words, or scenes, yielding a more ecologically valid stimulus set that mimics real-life experiences (Leal et al., Learning & Memory, 2019). We have developed several novel mnemonic discrimination tasks that manipulate stimulus similarity in order to tax hippocampal pattern separation (Leal, Tighe, & Yassa, NLM, 2014; Leal et al., Learning & Memory, 2016).
Translational applications of the pattern separation framework
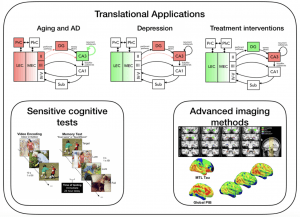
While disorders of mood and memory are often studied in isolation from one another, they implicate the same core vulnerability of the MTL system across conditions. Aging and depression share common cognitive and neurobiological changes such as impaired episodic memory, susceptibility to interference, and subtle MTL changes. While there is a great deal of overlap, each state of cognitive impairment yields a different cognitive and neurobiological profile that may be useful in targeting different aspects for treatment. Applying sensitive cognitive measures with concomitant high-resolution imaging allows us to tease apart subtle MTL differences in network functioning and connectivity in mood and memory disorders.
Depression
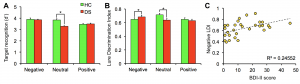
We applied one of our emotional mnemonic discrimination task to individuals with depressive symptoms and found that neutral discrimination was impaired but negative discrimination was enhanced. Further, negative enhancement in memory was positively correlated with symptom severity (Leal et al., NLM, 2014). Using high-resolution fMRI, we found decreased DG/CA3 activity, while activity in the amygdala was increased in individuals with depressive symptoms. This suggests that enhanced processing of negative information may be due to a network imbalance between the amygdala and the DG/CA3 (Leal et al., Hippocampus, 2014). Thus, DG/CA3 activity may be a useful biomarker to test the efficacy of drugs that may target the hippocampal system in depression.
Emotion regulation impacts on memory
Increased mnemonic discrimination for negative emotional content is associated with psychological disorders such as depression (Leal et al., NLM, 2014). Conversely, older adults typically demonstrate a positivity bias in memory, which has been associated with adaptive changes in emotion regulation (Mather & Carstensen, TICS, 2005; Ford, DiBiase, Ryu, & Kensinger, Psychol Aging, 2018). Thus, some our recent work has been exploring novel emotion regulation training approaches as possible mechanisms altering the encoding of emotional memories. We found that applying psychological distancing, a tactic of cognitive reappraisal, during encoding rescued the typical memory dysfunction in depression, evidenced by reduced negative affect as well as a reduced negative and enhanced neutral mnemonic discrimination during retrieval. These results suggest that cognitive emotion regulation strategies provide an effective approach toward altering both affect and memory in depression (Hayes et al., under review).
Aging
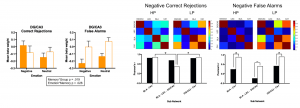
Episodic memory deficits are commonly reported in older adults (for review see Leal & Yassa, TINS, 2015). Interestingly, older adults show a bias towards remembering positive information (Mather & Carstensen, TICS, 2005). We found that older adults showed better emotional compared to neutral discrimination of similar items, a pattern that was opposite to what we found in young adults (Leal & Yassa, Behavioral Neuroscience, 2014). During high-resolution fMRI, we found signals consistent with emotional pattern separation in the DG/CA3 in cognitively normal older adults but not in those with subtle memory impairments. We also found a selective deficit in the amygdala-lateral entorhinal cortex—DG/CA3 network, which comprises a key pathway by which emotion modulates memory, during negative discrimination in age-impaired individuals and evidence of hyperconnectivity across the MTL during negative false recognition (Leal et al., Neurobiology of Aging, 2017). We have also found a specific activity profile for older individuals with late-life depression suggesting the amygdala-lateral entorhinal cortex—DG/CA3 network is specifically implicated in late-life depression (Leal et al., Hippocampus, 2017).
Menopause, memory, and risk of Alzheimer’s disease
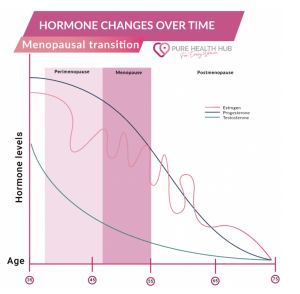
AD is significantly more prevalent and severe in females than males. Thus, a biological component such as menopause may underlie the differential impact of AD on females. However, current research regarding the impact of menopause on memory is insufficient, especially in the context of AD. Our ongoing project examines the effect of estrogen declines with menopause on medial temporal lobe network dynamics and emotional memory performance in aging females at greater genetic risk for AD. Using high-resolution MRI, a novel mnemonic discrimination task, and salivary assays, we will explore the interaction between the transition to menopause and the preclinical phase of AD as these events overlap during mid-life. This work will be an important step forward for filling the gaps in our knowledge of sex differences in the brain and towards the early detection of AD, which is crucial for intervening and slowing disease progression.
Detecting change early using high-resolution imaging and PET biomarkers
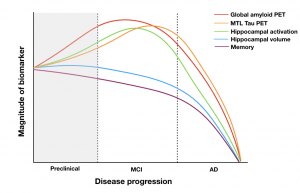
The cause of AD is still unknown. There are many hypotheses suggesting various sources might be contributing to the development of AD, such as amyloid, tau, or hippocampal hyperactivity. Failures of phase III clinical trials of anti-amyloid therapies have raised the possibility that such trials begin too late (Sperling et al., Sci Transl Med, 2011). Earlier detection of AD is critical to slowing down disease progression and to determine the initial cause of AD development. With the recent development of amyloid and tau PET imaging, we have examined AD pathology in cognitively normal older adults in vivo and can observe change in this pathology longitudinally. With the use of sensitive cognitive measures, high-resolution MRI, and PET imaging, we can enrich our understanding of normal versus pathological aging.
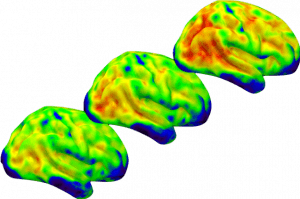
Current approaches to the early detection of AD rely upon classifying individuals as “positive” or “negative” for amyloid biomarkers. However, accumulation begins years before biomarkers become abnormal. In a recent paper, we used amyloid and tau imaging to investigate the earliest changes in AD pathology and found an inverted-U relationship between baseline amyloid levels and amyloid slope in asymptomatic older adults, suggesting a slowing of amyloid accumulation even in cognitively normal adults. Even in those who were nominally amyloid negative, the rate of amyloid accumulation and baseline amyloid predicted early tau deposition (Leal et al., J Neuro, 2018). In another study, we tested the hypothesis that hippocampal hyperactivity drives amyloid accumulation and found that greater hippocampal activation at baseline was associated with increased amyloid in cognitively normal aging (Leal et al., eLife, 2017). These results suggest we can detect changes in the brain very early on.